|
Understanding
Supercharging, Part 1
By Darren Mayer
Drawing and photos courtesy Kobelco and by Jeff
Burk
3/7/03
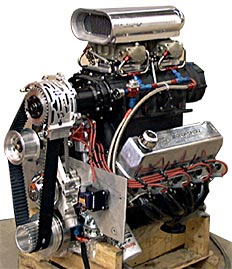 Supercharger engineer and designer Darren Mayer plies his trade at Kobelco designing and building superchargers. Over the years, he has been involved in building winning supercharged Pro Mod and Pro Street cars. His supercharged Nova Outlaw Street car was a two-time winner of the World StreetNationals. Mitch Stott used one of his Kobleco 'chargers on the Chrysler Hemi that powered his 'Vette to the first ever five-second doorslammer pass. Mayer owns Thundercraft Engines, which built the supercharged engine shown above, and Redline Designs. In addition, he is currently the crew chief for Johnny Rocca's new "Darkhorse" '51 Merc. Supercharger engineer and designer Darren Mayer plies his trade at Kobelco designing and building superchargers. Over the years, he has been involved in building winning supercharged Pro Mod and Pro Street cars. His supercharged Nova Outlaw Street car was a two-time winner of the World StreetNationals. Mitch Stott used one of his Kobleco 'chargers on the Chrysler Hemi that powered his 'Vette to the first ever five-second doorslammer pass. Mayer owns Thundercraft Engines, which built the supercharged engine shown above, and Redline Designs. In addition, he is currently the crew chief for Johnny Rocca's new "Darkhorse" '51 Merc.
This is the first of a two part series that will introduce the reader to all forms of mechanically driven compressed air devices. In the second installment Mayer will deal specifically with the advantages and disadvantages of using a supercharger by the sportsman and budget racer.
 |
Supercharged! The word sends the same
message to all gearheads around the globe.
Doorslammers around the world rely on
some form of supercharging to increase
the horsepower available to propel their
hot rods faster than could be expected
of any naturally aspirated engine. What
are the popular means of supercharging
today's doorslammers?
Superchargers are simply devices that
force air into an engine at a greater
rate than mechanically possible under
normal atmospheric conditions (thus the
term "blower"). If one considers supercharging
as a means of increasing the amount of
oxygen per intake cycle, then it is safe
to say supercharging can be done via mechanical
or chemical means.
|
CHEMICAL
ADVERTISEMENT

|
This
is a way to introduce a high pressure oxygen
liberating chemical into the intake stream resulting
in an increased oxygen content charge that is
super-cooled. Nitrous oxide is the popular door
car chemical that has been making power for
many years dating back to warplanes in WWII.
Nitrous oxide (N2O) is an inert gas that is
neither flammable nor breathable, as the oxygen
atoms are attached to a nitrogen atom. Until
the oxygen atoms are liberated, the gas is not
useful. After the oxygen atoms are broken from
the nitrogen atom (via heat) the oxygen can
be used as an ingredient in the combustion process.
Because of the increased oxygen, an increased
fuel quantity is needed to produce the increased
power and also to stop engine damage from an
oxygen-rich condition called running lean.
While nitrous oxide is impressive with the
amount of power it can increase to the engine's
power, it is not as effective as a mechanical
supercharger.
NEXT
PAGE >>
|